Department of Petroleum Engineering, UniveRsity of Tripoli, Tripoli, Libya
Received Date: October 12, 2016; Accepted Date: October 18, 2016; Published Date: October 25, 2016
Citation: Khazam M, Arebi T, Mahmoudi T, Froja M (2016) A New Simple CO2 Minimum Miscibility Pressure Correlation. Oil Gas Res 2: 120. doi: 10.4172/2472-0518.1000120
Copyright: © 2016 Khazam M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Oil & Gas Research
The minimum miscibility pressure (MMP) is one of the most important parameter to be determined in miscible gas injection projects to ensure and maximize the displacement sweep efficiency inside the reservoir. Usually the most effective way of determining the MMP is to run slim tube experiments. However, in the early screening stage, we often relay on the published empirical correlations to estimate the MMP and identify the candidate fields for EOR gas injection projects. The main objective of this paper was to examine different published empirical CO2 MMP correlations using measured data mainly obtained from Libya and other published resources, and also to develop a new simple reliable correlation to be applied in the oil industry. The data collected covered a wide range of CO2 MMP (1544-6244 psia) and oil API gravity (28-50ºAPI). Minitab regression tool was extensively used in our study and a wide range of new constructed correlations ranging from simple to complex ones were developed and statistically evaluated. The proposed simple CO2 MMP correlation is mainly function of the measured Pb, API, T and Rsi and has very reliable degree of accuracy (SD=6.7%, ARE=0.44%, AARE=5.74%, R2=95.22%) for the examined data and has shown better performance when compared with the industry popular correlations. The new correlation was validated against 100 measured PVT variables (Pb, Rsi, T and API) obtained from Libya, and the predicted CO2 MMP results have demonstrated very reliable trend (within the measured CO2 MMP trend) with no anomalies.
CO2 MMP; EOR Screening; PVT variables
Rsi: Initial Dissolved Gas Oil Ratio; Bo: Formation Volume Factor; ρo: Oil Density; μo: Oil viscosity; MW: Molecular weight; γg: Specific gravity; μ: Viscosity; Pb: Bubble Point Pressure; T: Reservoir Temperature; Y(c2-c6): Intermediate Components mole fractions of the oil MWC7+: Molecular weight of the C7+ Components; API: Stock-tank oil gravity; MMP: Minimum Miscibility Pressure; DL: Differential Liberation; CCE: Constant Composition Expansion; GOR: Gas Oil Ratio; ARE: Average Relative Error; AARE: Absolute Average Relative Error
Through research over the past 30 years, miscible phase displacement processes that use certain gases as inject agents have been developed as successful means for enhancing oil recovery from many reservoirs. CO2 is regarded to be an excellent solvent for miscible CO2 floods but still there are both advantages and disadvantages to take into consideration for applying CO2 EOR projects. MMP is defined as the minimum pressure that is required to attain the miscibility between an injected CO2 gas and oil at reservoir conditions. The MMP is the single most important parameter in the design of a miscible gas flood. A reliable estimation of the MMP helps the operator to develop injection conditions and to plan suitable surface facilities. In view of its importance, the operator is strongly advised to determine the MMP for site-specific candidate gasoil system under representative reservoir conditions [1]. CO2 MMP is usually determined by experimental approach. The experimental methods are time-consuming and expensive and are usually conducted when the company decide to proceed with the implementation of CO2 EOR project. On the other hand, empirical correlations are used to estimate the CO2 MMP and have their own limitations, though they are extremely useful for fast prescreening reservoir candidates for potential CO2 injection. Therefore, it is of principal importance to develop a reliable and accurate general correlation for determining the CO2 MMP for most of the worldwide crude oil. In the petroleum industry, the most widely used experimental methods are the slim-tube and the rising bubble apparatus. Slim-tube measurements are the preferred method for establishing MMP experimentally as both condensing and vaporizing effects can be captured discretely [2]. In several empirical correlations, different parameters that are mainly related to PVT properties, reservoir temperature, and oil composition have been considered as the most important variables that affect the MMP. For example, all the correlations in the literature suggest that the calculated MMP should increase with the reservoir temperature [3]. The early attempts for establishing CO2 MMP correlation was made by Holm and Josendal [4] in 1974, and was then extended by Mungan [5]. Their correlation requires the knowledge of the reservoir temperature and C5+ molecular weight of the reservoir oil. According to this correlation the effect of oil composition becomes more pronounced as temperature increases above the 120 to140°F. In 1978, Cronquist [6] proposed an empirical equation that was generated from a regression fit on 58 data points. Cronquist characterizes the miscibility pressure as a function of reservoir temperature, molecular weight of the oil pentanesplus fraction, and the mole percentage of methane and nitrogen. In 1979, Lee [7] has based his correlation on equating MMP with CO2 vapor pressure when T < CO2 critical temperature, while using the corresponding correlation when T > CO2 critical temperature. In 1980, Yellig and Metcalfe [8] proposed a correlation for predicating the CO2 MMP that uses the temperature, as the only correlating parameter, where system temperature (T) is in °F. Yellig and Metcalfe pointed out that, if the bubble-point pressure of the oil is greater than the predicted MMP, then the CO2 MMP is set equal to the bubble-point pressure. In 1985, Alston et al. [9] developed an empirically derived correlation for estimating the MMPs for pure or impure CO2/oil systems. Alston and coworkers used the temperature, oil C5+ molecular weight, volatile oil fraction, intermediate oil fraction, and the composition of the CO2 stream as the correlating parameters. Glaso [10] also, 1985, proposed a correlation for predicting minimum miscibility pressure of multi contact miscible displacement of reservoir fluid by hydrocarbon gases and CO2. His correlation is mainly function of reservoir temperature and molecular weight of C7+. In 1988, Eakin and Mitch [11], were observed the minimum miscibility pressures (MMP) for 102 combinations of oil, temperature, and solvents using Rising Bubble Apparatus. The data were represented with 4.5% standard deviation by an equation which needs only the solvent composition, oil C7+ fraction molecular weight, and the pseudo reduced temperature. A slightly better standard deviation of 3.5% was obtained by extending Peng’s procedure for critical points of mixtures to calculate MMP. Here in this paper the approach we have adopted for developing our correlation is based on the following key steps:
1. Establish a relation between measured CO2 MMP and only one selected variable to find out which of these variables has direct effect on minimum miscibility pressure (MMP) predictions.
2. Comingle the most effective variables together in different forms of equations and relate them with the measured CO2 MMP to find out the most suitable form of correlation that will provide a reliable accuracy of results.
3. Examine different forms of correlations ranging from simple to complicated form and highlight the features/limitations of each one and finally conclude the most reliable and practical correlation for use in the oil industry.
4. Test the correlations for their validity, quality and applicability against measured PVT data to ensure of no abnormal predictions. The correlations were tested against 100 PVT data points obtained from Libya.
5. Compare the new correlation with the most popular correlations to check its reliability.
Experimental CO2 MMP measurements were collected from different fields, mainly in Sirte basin of Libya [12], and from other worldwide literature data. A total number of 40 data points were initially obtained but some of the main related PVT parameters are not available in these data. Therefore, the actual number of data after screened were reduced to 20 data points to develop our correlation. However, when we examined the former industry correlations, different number of sample data points were used depending on the requirements of each correlations. Table 1 below describes the range of experimental data used in this research.
| Parameter | Range | ||
|---|---|---|---|
| CO2 MMP | psia | 2065 | 6224 |
| Solution GOR (Rsi) | SCF/STB | 162 | 1971 |
| Stock-tank Oil Gravity | oAPI | 32 | 50 |
| Reservoir Temperature | oF | 164 | 262 |
| Bubble-Point Pressure | psig | 645 | 3780 |
| Bubble-Point FVF | RB/STB | 1.141 | 2.320 |
Table 1: Range of experimental data used in this research.
It was our initial objective to test the possibility of finding a relationship between the measured CO2 MMP and a single independent variable that allows the user to calculate the MMP using one variable without the need for a relationship based on the use of more than one variable. The relations covered the following forms:
1. Relation between measured CO2 MMP and oil API gravity
2. Relation between measured CO2 MMP and Pb
3. Relation between measured CO2 MMP and MW C7+
4. Relation between measured CO2 MMP and T
5. Relation between measured CO2 MMP and Rsi
For the above examined variables and using Minitab regression function, different correlations were established ranging from linear (API) to quadratic relation (T and MWC7+) to cubic relation (Pb and Rsi). The criteria for classifying these correlations were based on the best fit as indicated by statistical means of Minitab standard deviation (SD), R-squared value, Sum square of errors (SS), mean square (MS). It should be pointed out that none of these relations could be adopted or considered reliable, as standalone, for CO2 MMP predictions due to high percentage of errors.
In most published empirical correlations, different PVT parameters were used as basic input parameters for CO2 MMP predictions. All these correlations in the literature [4-11] suggest that the calculated MMP should increase with reservoir temperature, while some of them apply different parameters to address the effect of the oil composition on MMP. Other empirical correlations predict CO2 MMP as a function of three variables; namely temperature, molecular weight of plus fraction, and the mole fraction of a light component in the reservoir oil. In our study we have adopted different approach than those available in literature and we have considered other PVT parameters such as API, Rsi and Pb that could contribute to the improvement of CO2 MMP predictions. Not only that, but also with the help of Minitab, we have tried to examine different forms of 4 parameter correlations until we achieved the best fit. After many trails we reached the below form of correlation (Equation 1). The chosen parameters are easy to measure at the wellhead and in the absence of Pb value, other correlations such as Standing [13] and or Khazam [14] can be used to predict this variable.
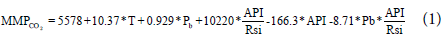
Where:
MMPCO2: Minimum miscibility pressure of CO2 solvent (psia)
T: Reservoir temperature (°F)
Pb: Bubble point pressure (psia)
Rsi: Initial solution gas oil ratio (Scf/Stb)
API: Oil gravity (°API)
A scatterplot of each independent variable with the CO2 MMP for this correlation are shown in Figure 1. It is clear that relatively all variables, as separate, have a trend except for API with more scattered relation. In the model building sequence section, Figure 2, shows that the value of R-squared is adjusted as the software try to add and multiply variables in the displayed order until it reaches the maximum possible value of R-squared.
The new developed correlation yielded a close to accurate prediction of the experimental CO2 MMP with R-squared equal to 95.22%, as shown in Figure 3 in the green side. Figure 4 shows a comparison between the CO2 MMP calculated with the above simple new correlation and the measured MMP data. Standard deviation was determined to be about 6.77%, average relative error equal to 0.44%, and the average absolute relative error determined to be about 5.74%. As outlined above, and to verify the reliability of the proposed new CO2MMP correlation, considering all statistical evaluation and indicators with the help of Minitab software, the correlation was tested across a wide range of measured variables. Due to the unavailable extra measured CO2 MMP experiments, therefore we adopted different approach to test the correlation against the measured variables and compare the trend of MMP prediction and find out if there is any anomalies behavior. This correlation was tested using approximately 100 measured PVT data points (Pb, Rsi, T and API) obtained from Libya [14]. The new correlation has demonstrated very reliable trend with no anomalies and all the predicted CO2 MMP are positive as it can be observed in Figures 5-8 through 8. Also when compared with the measured value (orange dots) are all within the overall prediction trend. The accuracy of the proposed new CO2 MMP correlation relative to the experimental data as well as other published correlations was computed using the following well known statistical means:
- Average Percent Relative Error,
- Absolute Average Percent Relative Error,
- Standard Deviation,
- R-Square,
- Sum of squares,
- Mean squared errors,
- F ratio
- Pearson correlation coefficient.
The results of statistical analysis, focusing on the top four indicators, were presented in Table 2. Our new correlation has demonstrated very reliable accuracy and is more superior to the other industry published correlations, tested for our data, with calculated standard deviation of 6.77%, average relative error of 0.44% and absolute average relative error of 5.74%. The industry published correlations demonstrated an absolute relative error ranges between 14.04% to 20.40% which are much higher percentage of error than our correlation and the standard deviation ranges between 14.67% to 29.52% which almost triple times of our correlation. Despite the limited number of data used to develop our correlation, but it covers a wide range of crude oil properties and a wide range of measured CO2 MMP. This gives the ability of our correlation to perform very well when compared with other industry published correlations and also provides high confidence on its applicability in the oil industry (Table 2). A graphical diagrams show a comparison between the calculated and measured MMP with the new correlation and other popular industry correlations using 45 line graphs, Figures 9-16 through 16 provide a better understanding of the reliability of the proposed simple correlation in compression to the industry published correlations asonecan see how the points are within a close fit of the line. Having established the simple correlation along with its parameters and statistical indicators, we turned efforts toward forming more complex correlation and see how contribution could be made for accuracy improvement. This complex correlation takes into consideration the effect of the mole fraction of the light components, and the molecular weight of the C7+, as additional variable parameters to the simple correlation form discussed above. All these parameters are comingled and tested with the help of Minitab to provide the complex correlation with following form, (Equation 2):
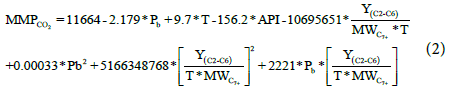
Where:
MMPCO2: Minimum Miscibility Pressure of CO2 solvent (psia),
T: Reservoir Temperature (°F),
Pb: Bubble Point Pressure (psia),
API: Oil API Gravity,
Y(C2-C6): Total Mole Fraction of Light Components,
MWC7+: Molecular weight of C7+ fraction.
| Statistical Mean % | Holm et al. Correlation | Yelling et al. Correlation | Cronquist’s correlation | Alston’s Correlation | Glaso Correlation | Eakin et al. Correlation | Lee Correlation | New Correlation |
|---|---|---|---|---|---|---|---|---|
| ARE | 3.66 | -11.22 | 13.31 | 3.63 | 0.18 | 14.92 | 13.00 | 0.44 |
| AARE | 14.10 | 15.82 | 18.08 | 22.79 | 14.04 | 20.40 | 19.91 | 5.74 |
| SD | 15.81 | 14.67 | 21.00 | 29.52 | 17.48 | 19.74 | 25.51 | 6.77 |
| R2 | 10.4 | 50.8 | 77.5 | 67 | 56 | 68.7 | 53 | 95.2 |
| Number of Data Points | 16 | 25 | 29 | 24 | 29 | 29 | 29 | 20 |
Table 2: Statistical analysis of CO2 MMP correlation.
Table 3 summarizes the complex correlation statistical indicators. The accuracy improvements over the simple correlation are negligible, where the SD is 5.2% compared to 6.7% and the AARE is 4.2% compared to 5.7% and R2 is 97% compared to 95%. These improvements are within the resolution of experimental errors and therefore practically will not justify the preference of this complex correlation over the simple correlation. On the contrary, the simple equation will receive more acceptance in the oil industry for its simplicity and ease of use and more important is the availability of correlation parameters that are easy to obtain compared to the above complex form.
| SD | R2 | R | ARE (%) | AARE (%) |
|---|---|---|---|---|
| 5.23 | 0.971 | 0.985 | -0.25 | 4.20 |
Table 3: Statistical analysis for complex correlation.
1. Twenty (20) data points, mostly collected from Libyan experimental data, were used to assess the widely used CO2 MMP correlations and also to develop a new correlation. The collected data covered a wide range of CO2 MMP (1544-6244 psia) and oil API gravity (28-50ºAPI).
2. A new simple CO2 MMP correlation, function of the measured Pb, API, T, and Rsi , was developed and has demonstrated very reliable degree of accuracy (SD=6.7%, ARE=0.44%, AARE=5.74%, R2=95.22%) for the examined data.
3. The new correlation is more superior to the other industry published correlations, examined in this study. The range of the published correlations errors (AARE and SD) are almost triple times of our new correlation’s accuracy.
4. Due to the unavailable other sources of measured CO2 MMP experiments, the new correlation was validated against 100 measured PVT variables (Pb, Rsi, T and API) obtained from Libya, and the predicted CO2 MMP results have demonstrated very reliable trend (within the measured CO2 MMP trend) with no anomalies.
5. Introducing more variables such as mole fraction of the light components and the molecular weight of the C7+ to the simple proposed correlation will add negligible improvement to the accuracy.
MMP predictions are very essential in the early screening stage of CO2 EOR candidate fields. The new proposed simple correlation can be reliably utilized in Libya to screen EOR field candidates for CO2 injection as well as can be utilized worldwide.
We would like to thank the members of Petroleum Engineering Department at the UniveRsity of Tripoli for their academic support.
Make the best use of Scientific Research and information from our 700 + peer reviewed, Open Access Journals